Con la ayuda de los datos obtenidos en INPRES, creamos una planilla y gráficos en excel, para organizarlos según nuestro criterio.
Hecho con Mayra Schliemann
martes, 28 de mayo de 2019
lunes, 27 de mayo de 2019
Sismos en la Argentina
Buscando información de la activiad sísmica en mi país me dí cuenta de que hay mucha más de la que la mayoria cree. Por eso, con Mayra hicimos un informe acerca del tema.
viernes, 10 de mayo de 2019
Liquid's properties
Some questions of my chemistry exam included:
+ How can the boiling point of a liquid be lower?
The boiling point of a liquid can be lower if the external pressure is also lower, since the vapour pressure would reach the same pressure at a lower temperature.
+ How can I sublimate water?
Water can be sublimated with a pressure lower than 4,6 mm of Hg adding to an initial temperature lower than 0°C, the temperature should be increased until it reaches 0,02°C , where the H2O will already be vapour.
The following diagram explains the phases of water and the balance points between them:
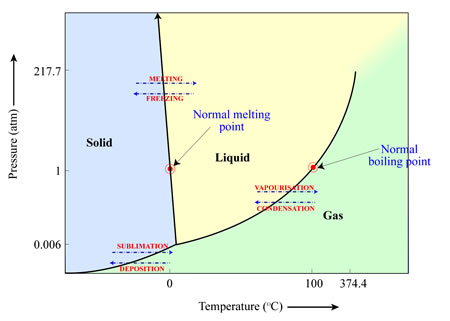
The line between solid and liquid shows the balance point between both states of water, and the arrows show that if the H2O we had, goes from the solid state to the gas state (without going through the liquid state), that transition would be called sublimation.
The following diagram explains the phases of water and the balance points between them:

The line between solid and liquid shows the balance point between both states of water, and the arrows show that if the H2O we had, goes from the solid state to the gas state (without going through the liquid state), that transition would be called sublimation.
+ What substance has more Surface Tention, water or naphtha?
Surface tension is a property of liquids, it is the dynamic balance that the pressure has on vapour against the walls of the container and the liquid. Water (H2O) has more surface tension that Naphtha (C8H18), since water has stronger intermolecular forces due to the fact that it contains Hydrogen Bond and Permanent Dipole, while Naphtha only has London Forces. Having stronger intermolecular forces means that molecules would be more difficult to separate, therefore to break their bonds on their surface would be more difficult, also causing their surface tension to be higher.
Suscribirse a:
Entradas (Atom)